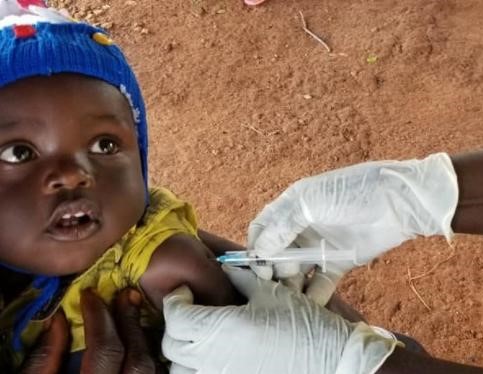
Health authorities and partners in Unity State on Tuesday launched a measles vaccination campaign after registering 50 cases of the contagious disease in displacement camps. Continue reading Measles-hit Unity State gears up vaccination
Category: Health
Go-Green company CEO denies ‘failing to keep Juba clean’
The Chief Executive Officer of the East Africa Go Green company has refuted Juba City Council’s allegations that the firm has failed keep the city clean – saying the municipal council closed dumping sites. Continue reading Go-Green company CEO denies ‘failing to keep Juba clean’
Surgeons embark on eye treatment campaign in Akobo
A national charity foundation has started a free eye surgery campaign for cataract cases in Akobo county of Jonglei State. Continue reading Surgeons embark on eye treatment campaign in Akobo
Specialist: Family planning prevents maternal deaths
A reproductive health specialist has emphasized the importance of family planning in attaining the social and economic well-being of families and their communities. Continue reading Specialist: Family planning prevents maternal deaths
Five drug dealers arrested in Hai Thoura
Juba City Council arrested five people suspected to be in possession of dangerous drug cocktails at the Thoura Residential Area on Thursday. Continue reading Five drug dealers arrested in Hai Thoura
Game-changing type 1 diabetes drug approved in US
A “game-changing” immunotherapy drug proven to delay the development of type 1 diabetes has been approved by regulators in the USA.
Experts say teplizumab marks a “new era” in treatment, tackling the root cause of the condition for the first time, rather than just the symptoms.
It works by reprogramming the immune system to stop it from mistakenly attacking pancreatic cells which produce insulin.
It is likely to pave the way for approval decisions in other countries.
About 8.7 million people have type 1 diabetes worldwide. In the UK the condition affects 400,000 people, including more than 29,000 children.
‘Taking away the burden’
In type 1 diabetes, the immune system (that normally fights off bacteria and viruses) mistakenly attacks key cells in the pancreas which produce insulin.
Insulin is crucial, helping the body use sugar for energy, and most current treatments focus on people checking their blood sugars and taking insulin – by injection or infusion – every day.
In 2019, a trial showed the drug delayed some people at high risk of the condition from developing it for an average of three years.
Experts say this delay can be very significant, particularly for young people who would not have to take daily insulin or monitor their sugars as intensively for that period of time.
They suggest people could also spend more years with their blood sugars in a healthy range, offering more time to be protected from the complications of high blood sugars such as kidney or eye disease.
Beth Baldwin’s son Peter died after a diabetic ketoacidosis emergency in 2014. He had undiagnosed type 1 diabetes and his body was shutting down. He was just 13.
Beth said: “A drug like this would be life-changing.
“You cannot stop people getting type 1 diabetes for now. But delaying the onset…. would be phenomenal – particularly for children.
“It means three years of not having to intensively manage the condition, and it may delay it long enough for more research to take place.
“It is a huge step forward.”
Beth now works with the charity JDRF UK to increase awareness of the signs of type 1 diabetes, including feeling very thirsty, urinating more than usual, feeling very tired, and losing weight without trying.
Rachel Connor, from the JDRF UK charity, which part-funded the trial, said: “This is a game-changer. To me, this is the start of a new era for the treatment of type 1 diabetes.
“It is the first time we are able to get to the heart of why the condition develops and help change the process, so we are not just treating the symptoms anymore.
“Once we can do that, we can find other ways to do it better and for longer.”
What is diabetes?
Diabetes is a lifelong condition that causes a person’s blood sugar level to become too high.
There are two main types:
- type 1 – where the body’s immune system attacks and destroys the cells that produce insulin
- type 2 – where the body does not produce enough insulin, or the body’s cells do not react to insulin
Type 2 diabetes is far more common than type 1.
Uganda set to receive Ebola trial vaccine
The head of the World Health Organization, Tedros Adhanom Ghebreyesus, says the first doses of a new trial vaccine for Ebola will be sent to Uganda next week.
Dr. Tedros said a clinical trial program would test whether the jab was effective against a Sudanese variant of Ebola.
He said three separate candidate vaccines had been evaluated by a committee of external experts.
The Ugandan authorities say they’re starting to succeed in their efforts to contain the current outbreak.
At least 55 people have died.
Dr. Tedros said an additional 22 people had been assessed as probable deaths.
Seventy-three patients have recovered from Ebola infection.
Doctor urges expectant mothers to visit antenatal care
A health expert is encouraging expectant mothers and their husbands to visit hospitals for antenatal services to lessen the risks of childbirth complications. Continue reading Doctor urges expectant mothers to visit antenatal care
Woman dies of unattended labor at Yambio hospital
Health authorities in Western Equatoria State said an expectant mother died from unattended labor complications at a hospital in Yambio, days after the only maternity ward at the facility was shutdown due to funding shortage. Continue reading Woman dies of unattended labor at Yambio hospital
Cyber security ‘not taken seriously’, warns ICT expert
A South Sudanese cyber security analyst has said individuals and organizations are not taking cyber security within their practices seriously enough. Continue reading Cyber security ‘not taken seriously’, warns ICT expert